Lecture 1.104- Reference Electrode
Imagine a glass of water, and an ice cube. What can we say about the temperature of the water, it’s hotter (with respect to the ice cube) but similarly, But when we have some hot soup near it the same glass is colder (with respect to soup thins time).


 Think, Water is hot or cold…
Think, Water is hot or cold…
This particular concept of taking a reference of some standard is something that will come again and again in every field because this is how we stick to number and relating it to some physical quantity, so stick to it, this is something very important and useful true science learner.
Similarly different electrode has difference numbers of electron in solution, at different concentration and surrounding conditions, and we can’t give a value to all of them without a testing every time.
There we take a Reference Electrode where we assume the electrode potential to be zero and then give numbers to rest comparatively, The first and primitive Reference Electrode we used was,
Standard Hydrogen Electrode (SHE)
Construction – what we discussed till this point, were solid electrodes but with exactly the same principle we have gaseous electrode as well.
In SHE, Hydrogen gas is passed and which is absorbed by the Pt (platinum) and rest of excess is passed through the solution in atmosphere.
The reaction with the absorbed hydrogen gas is same as any other metal, the redox equilibrium happens
2H+ + 2e– ——- > H2 (g)
Here this is only one electrode which we assume to have zero electrode potential reduction or oxidation, which makes it sound like it doesn’t have any tendency to reduce or oxidize.
So using Nernst equation or simply if we say that there is no contribution of a reference electrode we can conclude that Ecell is due to the other cell.
The next interesting this is that, the electrode potential depends on a lot of other things, so for a standard reference we have to fix everything that can vary the E0 of the electrode .From Nernst equation we can conclude on
- Concentration – Now as we know from the need of salt bridge concept that how the concentration of participating ion can alter the Electrode potential, so we need to fix the concentration of H+ ion in the solution.
- Anything that changes K – Now the good thing here is the K (equilibrium constant) of the cell reaction depends only on temperature and atmospheric pressure since this is a gas electrode.
Temperature – Absolutely it depends on the reaction to reaction, but we don’t care how it changes, it changes with temperature and so does the electrode potential, so fix temperature.
Pressure – The pressure change will make a change in K, plus we can see this as what amount of Hydrogen stick to Pt depends on what pressure the gas is applied on the Pt, So fix the pressure also.
Pt, H2 (1atm)|(1.0 M HCl)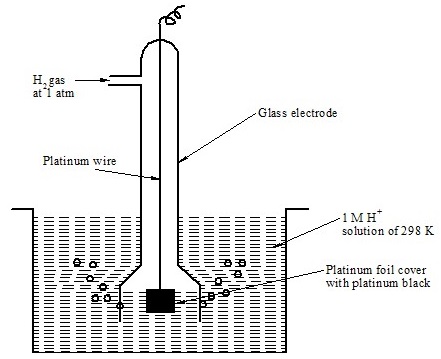
These are the conditions, and this electrode has zero electrode potential, An ideal Reference electrode.
Problems with the SHE (no offence, but this just a coincidence) –
- The hydrogen electrode is readily affected by compounds of Hg, As, S and agents some agents so the electrode don’t work properly in their presence.
- It cannot be used in solution containing redox systems(explained later)
- The most important, and something that makes a lot more sense is that the condition, of maintaining concentration and pressure throughout the process it not quite possible every time everywhere.
Now, since we have problems with the structure and we want this kind of electrode, what can we do(think about what chemical and physical properties you can wish for and how can we acquire those)?
Abhishek kumar jha
(Chemistry at Utkarshini)
notes to downlord are –1-104-electrochemistry-reference-electrode

